Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure. The chemical symbol for Iron is Fe. Neutron Number and Mass Number of Iron Mass numbers of typical isotopes of Iron are 56; 57; 58.
Helium. Helium in Periodic Table | by Chemistry Topics | Medium
The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons. As will be discussed in more detail later in this chapter, atoms (and molecules) typically acquire charge by gaining or losing electrons.

Source Image: pyramidalcube.blogspot.com
Download Image
Get Basic Information About Elements You’ll need to gather basic information about the elements to find the number of protons, neutrons, and electrons. Fortunately, all you need is a periodic table . For any atom, what you need to remember is: Number of Protons = Atomic Number of the Element Number of Electrons = Number of Protons

Source Image: numerade.com
Download Image
What do Mechanical & Methods Engineers need to know about atomic structures?
That’s where atomic number and mass number are useful. Figure 6.2.1 6.2. 1: It is difficult to find qualities that differ between each element, and to distinguish one element from another. Each element, however, does have a unique number of protons. Sulfur has 16 protons, silicon has 14 protons, and gold has 79 protons.

Source Image: doubtnut.com
Download Image
How Many Protons And Neutrons Does Iron Have
That’s where atomic number and mass number are useful. Figure 6.2.1 6.2. 1: It is difficult to find qualities that differ between each element, and to distinguish one element from another. Each element, however, does have a unique number of protons. Sulfur has 16 protons, silicon has 14 protons, and gold has 79 protons.
The mass number (A) is defined as the total number of protons ( p+ p +) and neutrons ( n n) in an atom: mass number = A = p+ + n (2.2.2) (2.2.2) m a s s n u m b e r = A = p + + n. Atoms of the same element always have the same number of protons, same Z, but often have different numbers of neutrons, therefore, different mass numbers.
The nucleus of Iron atom contains 26 protons and 30 neutrons. Find its
Therefore, an iron atom has twenty-six protons and twenty-six electrons. The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons. The difference between the mass number of the iron atom and the number of protons is thirty. Therefore, an iron atom has thirty neutrons.
Section 2.4 – Stellar Nucleosynthesis and Degenerate Matter – Astronomical Returns
Source Image: astronomicalreturns.com
Download Image
Calculate the number of protons, electrons, neutrons in Bromine with atomic number 35 and mass number 80.
Therefore, an iron atom has twenty-six protons and twenty-six electrons. The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons. The difference between the mass number of the iron atom and the number of protons is thirty. Therefore, an iron atom has thirty neutrons.

Source Image: toppr.com
Download Image
Helium. Helium in Periodic Table | by Chemistry Topics | Medium
Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure. The chemical symbol for Iron is Fe. Neutron Number and Mass Number of Iron Mass numbers of typical isotopes of Iron are 56; 57; 58.
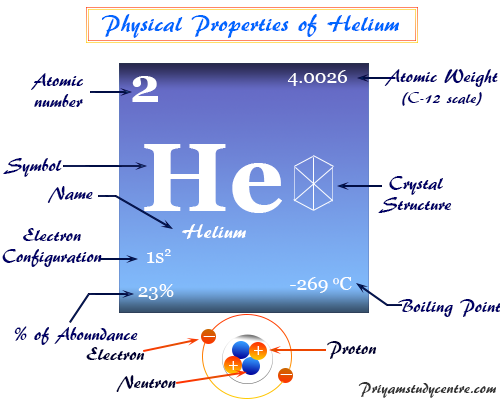
Source Image: medium.com
Download Image
What do Mechanical & Methods Engineers need to know about atomic structures?
Get Basic Information About Elements You’ll need to gather basic information about the elements to find the number of protons, neutrons, and electrons. Fortunately, all you need is a periodic table . For any atom, what you need to remember is: Number of Protons = Atomic Number of the Element Number of Electrons = Number of Protons

Source Image: m-cets.co.uk
Download Image
Protons, Neutrons, Electrons for Iron (Fe, Fe2+, Fe3+)
Answer – A neutral atom of iron has 26 protons, 30 neutrons, and 26 electrons. These numbers vary when iron is in any of its ionic or isotopic forms. Iron, which belongs to Group 8 of the periodic table, is a transition metal.
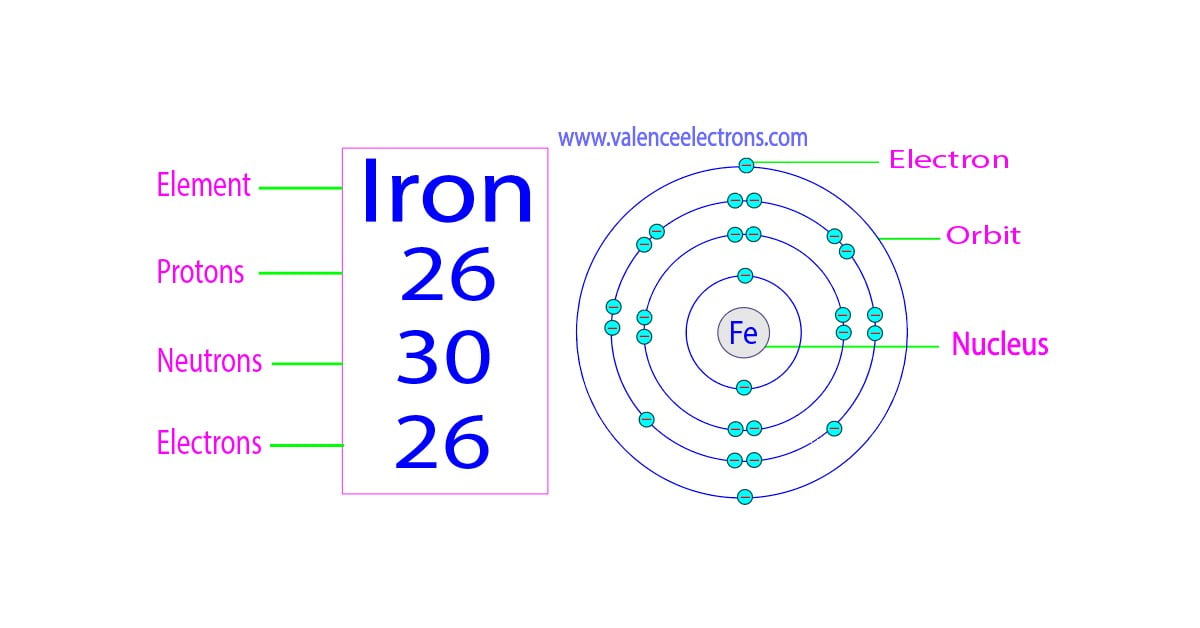
Source Image: valenceelectrons.com
Download Image
iron « KaiserScience
That’s where atomic number and mass number are useful. Figure 6.2.1 6.2. 1: It is difficult to find qualities that differ between each element, and to distinguish one element from another. Each element, however, does have a unique number of protons. Sulfur has 16 protons, silicon has 14 protons, and gold has 79 protons.

Source Image: kaiserscience.wordpress.com
Download Image
The Cosmic Gift Of Neutron Stars: A Live-Blog Event
The mass number (A) is defined as the total number of protons ( p+ p +) and neutrons ( n n) in an atom: mass number = A = p+ + n (2.2.2) (2.2.2) m a s s n u m b e r = A = p + + n. Atoms of the same element always have the same number of protons, same Z, but often have different numbers of neutrons, therefore, different mass numbers.

Source Image: forbes.com
Download Image
Calculate the number of protons, electrons, neutrons in Bromine with atomic number 35 and mass number 80.
The Cosmic Gift Of Neutron Stars: A Live-Blog Event
The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons. As will be discussed in more detail later in this chapter, atoms (and molecules) typically acquire charge by gaining or losing electrons.
What do Mechanical & Methods Engineers need to know about atomic structures? iron « KaiserScience
Answer – A neutral atom of iron has 26 protons, 30 neutrons, and 26 electrons. These numbers vary when iron is in any of its ionic or isotopic forms. Iron, which belongs to Group 8 of the periodic table, is a transition metal.